Imagine designing a life-saving device for a heart no bigger than a walnut.
At the Design of Medical Devices conference in April, Dr. John Criscione, Dr. Balakrishna Haridas and research associate/Ph.D. student Achu Byju from the Texas A&M University Department of Biomedical Engineering unveiled preclinical medical device designs aimed at treating pediatric heart deformities in infants and unborn fetuses.
The University of Minnesota hosted the pediatric sessions at the conference in partnership with the Southwest-Midwest Pediatric Device Consortium (SWPDC), an organization funded by the Food and Drug Administration to develop pediatric medical devices. The SWPDC is led by Texas Children’s Hospital, which serves as the clinical lead, and Texas A&M, which serves as the academic engineering lead.
Criscione, a professor of biomedical engineering, and Dr. Erica Hord Perez, a former Texas A&M student, are developing a biventricular compression implant to support infants recovering from complicated heart surgeries. When complete, the device could eliminate the need for prolonged use of extracorporeal membrane oxygenation (ECMO) devices: large external machines that can function as an artificial heart and lungs by pumping and oxygenating a patient’s blood outside of the body.
ECMO devices are regularly used during heart surgery with minimal side effects. However, when recovery is slow and the machines are used for extended periods of time, the possible complications are often deadly.
ECMO machines have a massive surface area, which increases the risk of life-threatening complications such as blood clots, leaks and infections. Complicating things even further, the patient's heart remains inactive while attached to these machines, something that healthy heart tissue never experiences.
“There's just an enormously high complication rate,” Criscione explained. “The survival rate is less than 50%.”

Criscione and Perez are working to improve these odds by taking an entirely different approach using an internal compression device placed around the patient's heart. By assisting the heart from the outside, the device would work with the patient’s natural system, rather than bypassing it. Because it does not come in direct contact with the bloodstream, the device has the potential to decrease clots, leaks and infections that often accompany external machines.
Criscione is also designing a similar device for adults but adapting it for infants has proved an extreme challenge.
"When it came to the pediatric device, we had to throw out the existing folding mechanism and come up with a different mechanism to bring all the parts together that was much more collapsible," he explained.
The result is a compact design that can fold in on itself and be inserted through a single access point, eliminating the need for risky open-heart surgeries. Once the patient is fully recovered, the device will fold on itself again, allowing it to be removed without surgery.
“You’re not making any more incisions, you’re just pulling it out through the port that it’s accessing,” Criscione said.
Byju and Haridas are focused on congenital heart defects that are diagnosed even earlier at the fetal stage. They are collaborating with Texas Children’s Hospital’s Fetal Center to develop miniature surgical devices for Hypoplastic Left Heart Syndrome (HLHS), a rare disorder where the left side of a fetus’s heart fails to develop properly. Infants born with it require multiple surgeries to survive.
Byju and Haridas’ work target an even narrower group: fetuses with HLHS who also lack the foramen ovale, the natural opening that allows blood to flow between the two sides of the fetal heart. For these patients, blood travels to the lungs and returns to the heart with nowhere to go, causing pressure to build and blood to back up into the still-developing lungs.
The damage is so severe that less than 25% of affected fetuses will survive birth.
If the appropriate minimally invasive tools are available, surgeons can operate directly on the fetus and create an artificial opening where the foramen ovale would be. On its own, the new opening will not correct the malformed heart, but it will allow more regular blood circulation, prevent lung damage and allow the lungs to develop properly, thus increasing chances of survival at birth.
“What we’re trying to do is get these kids to a point where they can have that first surgery,” Byju explained.
However, the pliability of fetal heart tissue means that such surgically made openings easily reseal. To keep this opening from closing, surgeons will implant small devices to hold the heart tissue in place.
Because of the low number of patients, no devices designed for fetuses are manufactured. Current implants are repurposed adult devices designed for entirely different procedures. In the fetal heart, these devices frequently shift out of place, undoing the benefits gained by the surgery.
“They do the best that they can,” Byju said. “But it’s not great outcomes.”
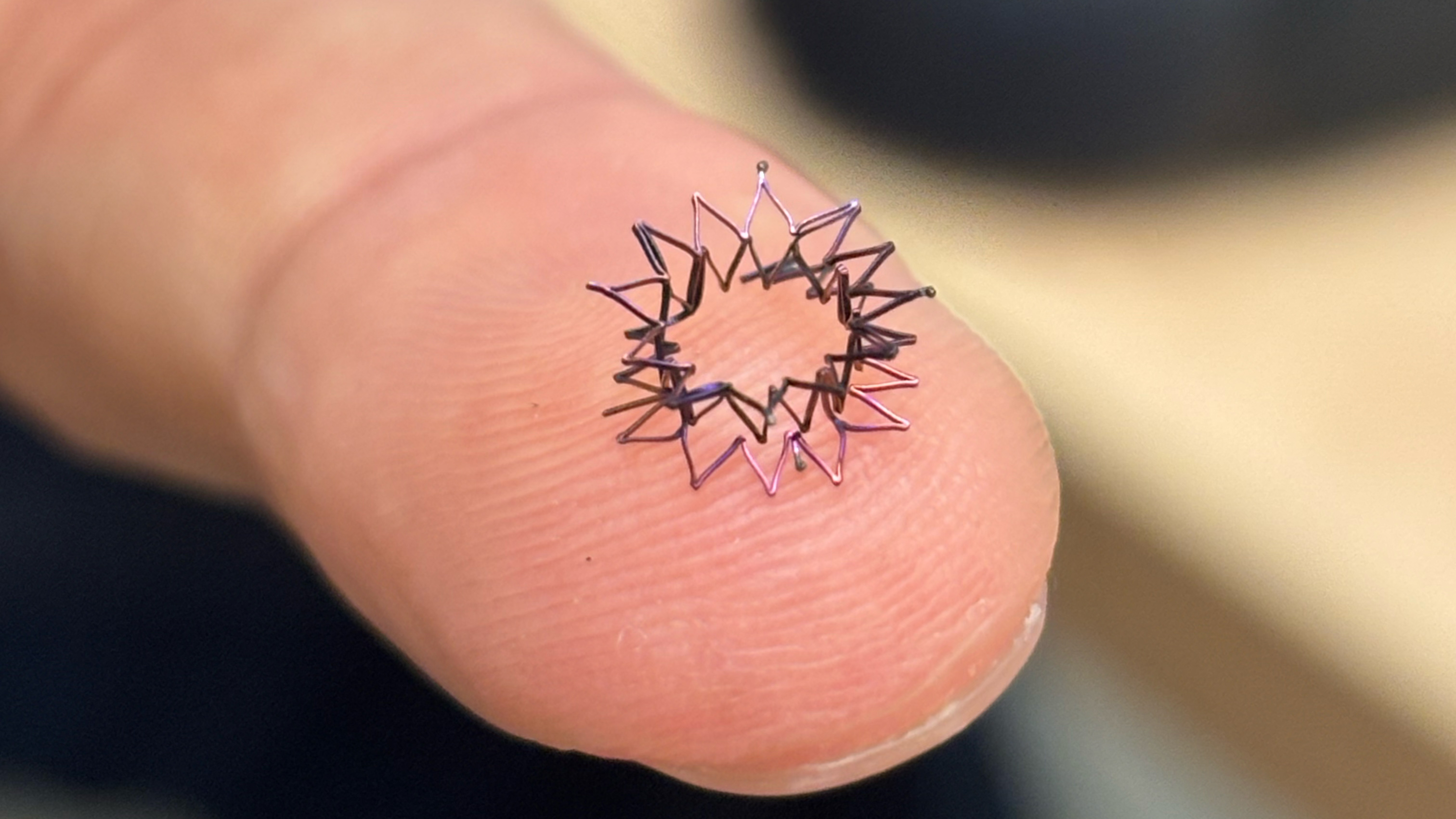
Byju, Haridas and Texas Children’s Hospital collaborators hope to change this. Researchers at Texas Children's Hospital designed a family of expanding hourglass-shaped wire mesh devices to secure the opening in a fetal heart and remain in place. While promising in concept, the designs may not generate enough force to stay anchored in the highly elastic fetal heart tissue without collapsing.
That's where Byju steps in. Using a combination of computer simulation and hands-on fabrication, he created a proof-of-concept design that can fit on a fingertip. He abandoned the hourglass shape in favor of a short tube with flanges on both ends to provide enough force to hold fetal heart tissue in place while maintaining a size significantly smaller than both the adult devices currently being used off-label, and Texas Children's Hospital's hourglass prototypes. The device collapses to be inserted using an 18-gauge needle with an internal diameter less than 0.04 inches (roughly 1 mm).
The device is only one possible solution to the problem: Byju and Haridas are also exploring alternative options to prevent the edges of the surgically created opening from sealing shut. Texas A&M has already filed a patent for another one of their potential solutions — a thermal device that could seal the edges of an opening in fetal heart tissue using controlled application of heat without requiring any implant at all, the ideal solution for mother, fetus and the surgical team.
Work continues on both approaches. Regardless of which disorder is being treated, or which solution is being pursued, the goal at Texas A&M’s biomedical engineering department remains the same: saving the small lives that would otherwise be lost.