The first glucose self-monitoring system created in 1970 weighed three pounds, was initially designed only for physicians’ offices and needed a large drop of blood for a reading. Over 50 years later, researchers at Texas A&M University are working to create a fully injectable continuous glucose monitor (CGM) so small it rivals a grain of rice and can be used with an external optical reader to measure sugar levels at any given time.
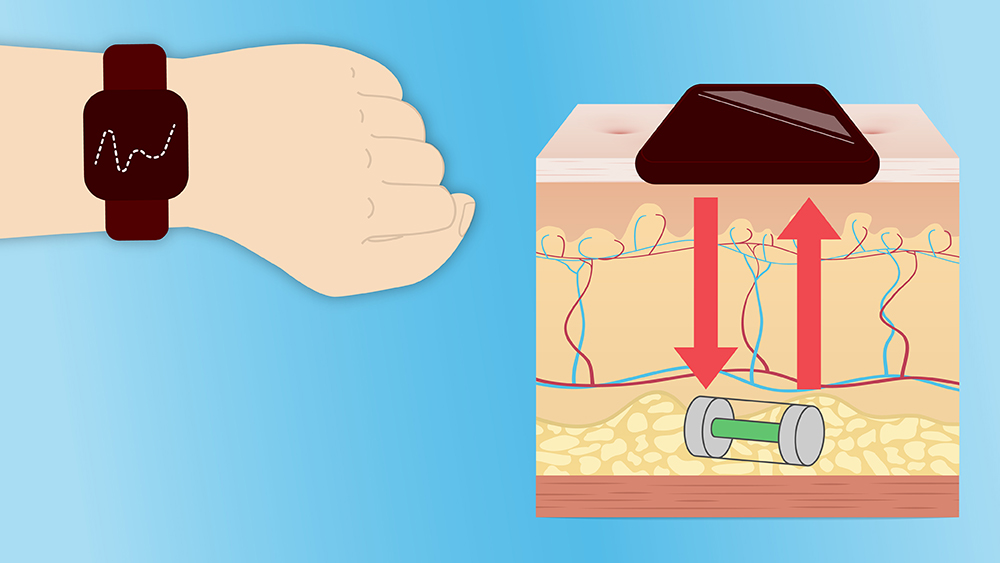
While CGMs have advanced over the last 25 years, current models can still be a nuisance to the user and the required upkeep may discourage use. To address this issue, two faculty members from the Department of Biomedical Engineering have received a National Science Foundation (NSF) grant to fund a multidisciplinary project to develop an injectable, grain-of-rice-sized glucose biosensor and wearable device enabling user-friendly, minimally-invasive continuous monitoring.
“CGMs are commercially available, but most of them are indwelling, meaning there is a needle in the skin connected to a patch on the arm,” co-principal investigator and Regents Professor Dr. Gerard Coté said. “There is one CGM that is totally implantable, but it is much bigger than ours and requires a doctor to surgically implant it with an incision.”
The NSF grant is backed by the biosensing program, a part of the engineering biology and health cluster of the NSF Chemical, Bioengineering, Environmental and Transport Systems (CBET) Division. The division aims to support trailblazing research and education in these areas.
Using autofluorescence to read blood sugar
Coté and his lab are designing the injected sensor’s chemistry and developing the watch-type reader device. The sensor is put under the skin and analyzed using light from the watch-like device to determine the glucose concentration. The reader sends the signal to a cell phone and the patient can share the results with their health provider.
“The chemical assay in the injectable sensor is used to determine the concentration of the glucose within the tissue, and the watch device sends in light and measures the fluorescence from the sensor to give the sugar concentration,” Coté said.
Aside from its unique size and injectability, the sensor and wearable reader employ an optical sensing technology that addresses the challenges associated with biosensing for populations of darker skin tones.
“We use chemistry that has a fluorescent color that emits in the red and infrared range rather than green light, which works better for the darker skin tones,” Coté said.
Protecting the sensor
Co-principal investigator Dr. Melissa Grunlan, Charles H. and Bettye Barclay Professor in Engineering, is working with her lab to wrap the sensing chemistry in a membrane that makes it more compatible within the body.

“When you implant something, the body thinks, ‘This does not belong here,’ and it tries to seal it off,” Grunlan said. “When that happens, that sensor can't work because there's no glucose getting into it. Our membrane is a hydrogel, similar to the material used for contact lenses, but this is a special hydrogel that is thermoresponsive.”
The thermoresponsive nature allows the sensor to sit subtly in the body. The surface actively swells and shrinks just enough to keep cells and proteins from sticking to the sensor and forming obstructive scar tissue around it.
Grunlan and Coté recently had a patent issued on the membrane technology and are considering different devices it could be used with, particularly to sustain the lifetime of medical devices.
“The membrane could be applied to assorted devices that are prone to adhesion processes in the body,” Grunlan said. “It could be used on maybe a catheter to prevent thrombosis and infection by preventing the accumulation of proteins, cells, and organisms.”
Multidisciplinary research in biomedical engineering
The project culminates nearly two decades of research and collaboration between Coté’s biosensing expertise and Grunlan’s biomaterial expertise.
“Working together as a team with complementary expertise is the key to developing complicated technologies such as this one,” Coté said. “Being able to collaborate with an expert like Dr. Grunlan in designing and developing biocompatible materials and bringing our experience in optical biosensor design is needed and gives us hope for success.”
Although the grant is new funding, Grunlan and Coté have been preparing for this stage of their project for some time now and feel confident in the device’s outcome.
“Dr. Coté and I have done extensive research in the past together that has led up to this study and this particular grant,” Grunlan said. “We've done some preclinical studies to assess the membrane's biocompatibility. He's done extensive work developing the sensing chemistry. What’s great about this grant is now we can prove our idea, and we’re in a really good position to do that.”