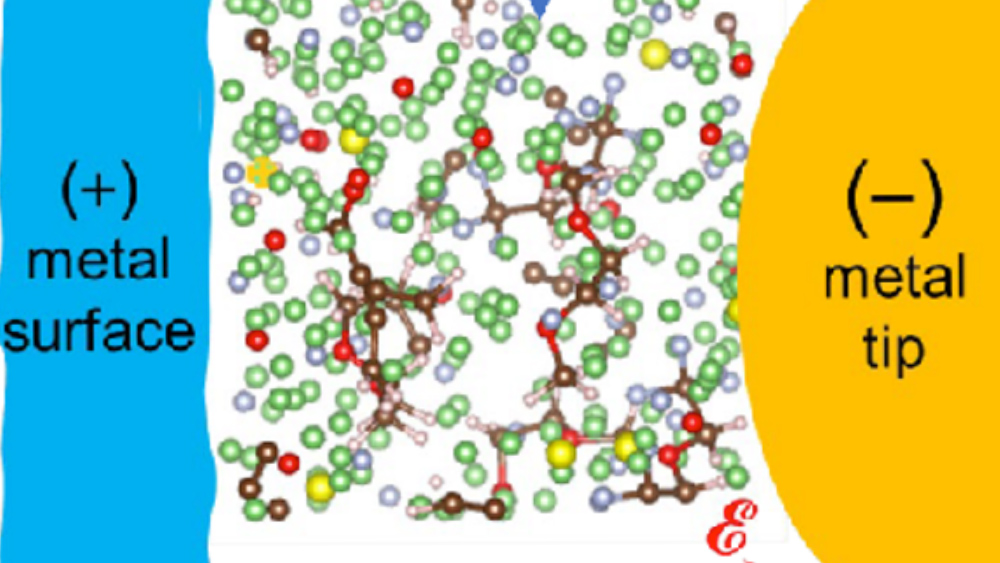
Researchers from the Artie McFerrin Department of Chemical Engineering at Texas A&M University, in collaboration with a team from Pacific Northwest National Lab (PNNL), have discovered how the solid–electrolyte interphase critically governs the performance of rechargeable batteries.
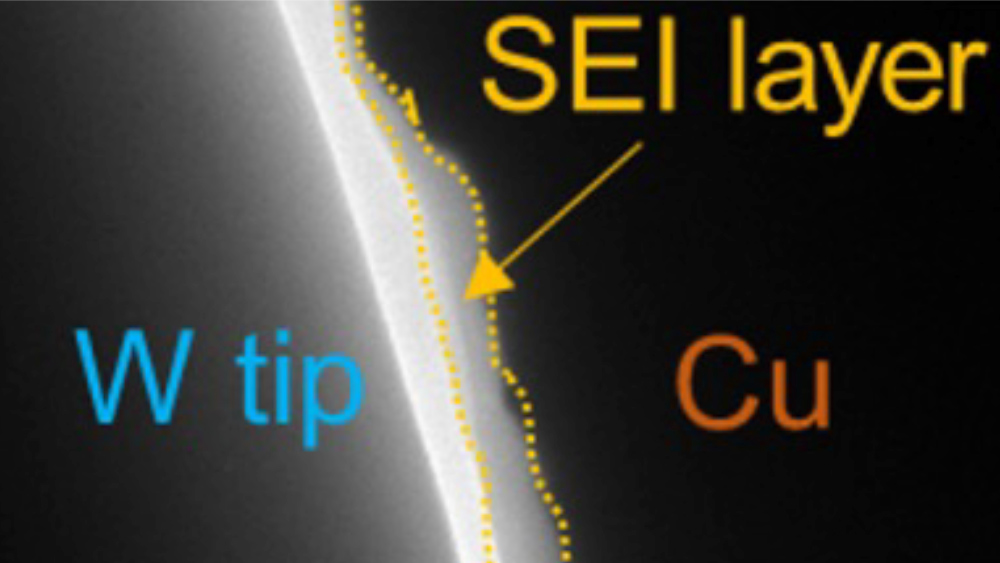
According to research recently published in Nature Energy, Dr. Perla Balbuena and Dr. Jorge Seminario describe their theoretical predictions that explain results from an in situ bias transmission electron microscopy (TEM) approach to directly measure the electrical properties of solid–electrolyte interphase (SEI) layers grown on copper and lithium substrates.
“Our work is to elucidate the properties of what is called a solid–electrolyte interphase interfacial film that forms on electrode surfaces in lithium metal batteries,” Balbuena said. “With our theoretical analysis, we revealed the correlation between the film structure and its electric properties for the first time.”
The SEI is an “unknown” material formed between the Lithium-metal (Li-metal) anode and the electrolyte during the first few cycles of a new rechargeable battery, Seminario said.
“Similar to what our colleagues at Pacific Northwest National Lab did experimentally, I took samples of the SEI formed on the surface of the Li-metal as calculated by Prof. Balbuena’s group,” Seminario said.
Using the calculated SEIs chemistries and morphologies, Seminario’s group predicted their current-voltage characteristics using a powerful technique based on the Schrödinger equation under non-equilibrium conditions.
“Here we use in situ bias transmission electron microscopy to directly measure the electrical properties of SEIs formed on copper and lithium substrates,” according to the article. “We reveal that SEIs show a voltage-dependent differential conductance.”
Although an ideal SEI is expected to be electrically insulative to prevent persistently parasitic reactions between the electrode, electrolyte and ionically, the true nature of the electrical properties of the SEI remained unclear due to the lack of a direct characterization method, according to the article.
“The electron transport technique (GENIP) has been fully developed in my group since the beginning of the nanotechnology era and the resulting electrical characteristics provided by our procedure can be directly compared to experiments to find out the nature of the SEI,” Seminario said.
From the point of view of fundamental quantum physics, this is an extraordinary piece of research that bridges the gap between theory and experiment. These advances are necessary, especially in our efforts to maintain a livable environment.
Overall, knowing the electrical properties of the SEI, such as the electron tunneling or leakage from the anode to the electrolyte, will lead to optimized electrolytes that will improve the quality and produce more powerful batteries for electric vehicles.
“The application of fundamental principles of quantum theory, such as electron transport, electron tunneling and the like are becoming a key point to develop synergistic research of quantum theory,” Seminario said.
According to the article, the function of a rechargeable battery depends on the synergy of three major components in the cell: the anode, electrolyte and cathode.
“The electrolyte, in either solid state or liquid state, is sandwiched between the cathode and the anode to facilitate ion transport,” according to the article. “For better battery performances, the SEI is expected to possess electrically insulative, ionically conductive and constant thickness properties.”
According to the article, these three characteristics are interactively correlated, and the electrical properties of the SEI control the SEI thickness.
The thickness of the SEI continuously increases during charge–discharge cycling and shelf storage, indicating that the SEI does not behave as an electrical insulator.
Overall, the research showed that the theoretical and experimental results followed the same trends and provided a way to determine which are the best electrolytes to use for new Li-metal batteries, Seminario said.
“From the point of view of fundamental quantum physics, this is an extraordinary piece of research that bridges the gap between theory and experiment,” Seminario said. “These advances are necessary, especially in our efforts to maintain a livable environment.”
Through this study, the team was able to gain a clearer understanding of how the electrolyte solution’s chemistry plays a significant role on the SEI properties, which influence the battery life, Balbuena said.
“By developing new understanding, we can develop ways of controlling the battery degradation phenomenon that is also very relevant to everyday applications,” she said.