
Energy demands are at an all-time high, as is the need to be environmentally conscious. That’s why chemical engineering professors Drs. Joseph Kwon and Mark Barteau have developed a strategy to predict the performance of new catalysts for greener ammonia production.
“By making industrial processes like ammonia production more sustainable, this research contributes to broader efforts to combat climate change, promoting a healthier planet for future generations,” Kwon said.
Changing how ammonia is produced has environmental and economic benefits and enhances food security. This can be achieved by lowering the energy requirements and carbon emissions associated with traditional ammonia production.
It is estimated that 1 to 2% of global energy consumption, and a similar fraction of man-made greenhouse gas (GHG) emissions, are associated with the production of ammonia for fertilizer.
Ammonia is produced by the Haber-Bosch process, a century-old technology that uses atmospheric nitrogen and hydrogen to produce ammonia. The hydrogen is typically generated from methane via a process that generates a large GHG footprint.
According to Kwon and Barteau, their research focuses on a greener alternative to the Haber-Bosch process. They are developing electrocatalysts for the electrochemical nitrogen reduction reaction, called NRR, using the hydrogen atoms in water.
“The goal is to understand and enhance the interaction of nitrogen and hydrogen on the catalyst surface to efficiently produce ammonia at lower temperatures and pressures,” Kwon said. “To make these catalysts work as we hope, we computationally analyze various materials and their properties to predict which will perform best under NRR conditions.”
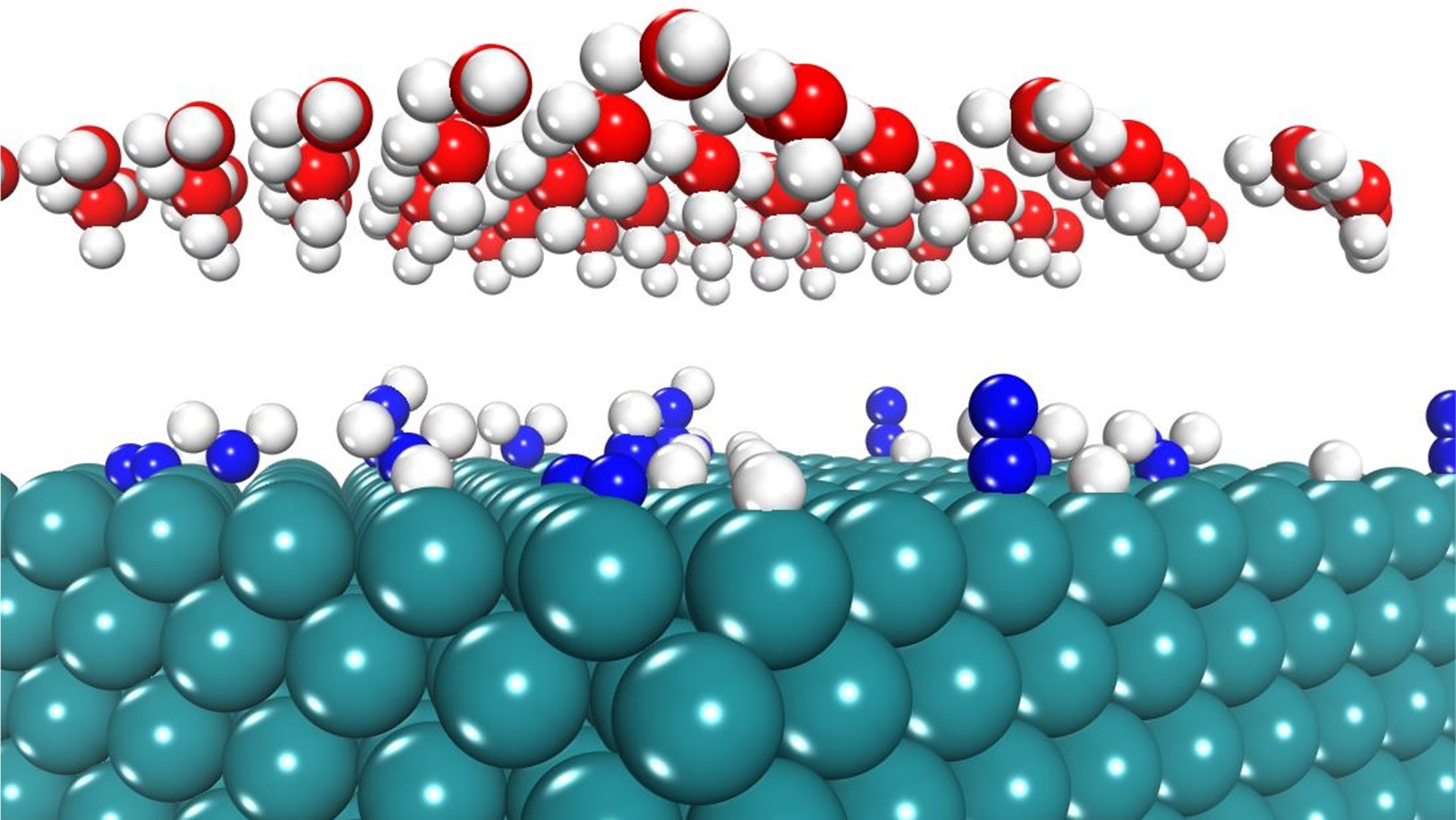
Kwon and Barteau’s team combines the computational methods of density functional theory (DFT) and kinetic Monte Carlo (kMC) simulations to predict the efficiency and the stability of catalysts under electrochemical reaction conditions.
Density functional theory is a modeling method that uses physics, chemistry and materials science to investigate the electronic structure of atoms and molecules. This method allows Kwon to predict how different materials will interact with nitrogen and hydrogen molecules.
“Computational tools like DFT and kMC have revolutionized catalyst design in the 21st century,” said Barteau, one of the early practitioners applying DFT to heterogeneous catalysts. “What is particularly powerful about the combination of these tools is the ability to predict the dynamic performance of active materials under different conditions and over time.
Kinetic Monte Carlo simulations use probability estimates to predict possible outcomes for an uncertain event. This will help researchers understand how the reactions evolve at different temperatures and pressures, providing insights into the scalability and lifetime of potential catalysts.
“We aim to explore new electrocatalyst materials,” Kwon said. “However, employing each method independently can limit the scope of properties regarding materials and reaction processes. This approach allows us to systematically optimize the catalysts before they are even tested in a laboratory, streamlining the development process and paving the way for more sustainable fertilizer production methods.”
Kwon also hopes that this research can lead to breakthroughs in catalyst design and a greater understanding of their function at the molecular level.
Several key areas that the research focuses on are reducing environmental impact, advancing green chemistry, promoting energy security and catalyzing technological innovation.
“This integrated approach not only accelerates the discovery of viable catalysts but also provides a comprehensive understanding of their behavior in real-world conditions, setting the stage for experimental validation and eventual industrial application,” Kwon said.