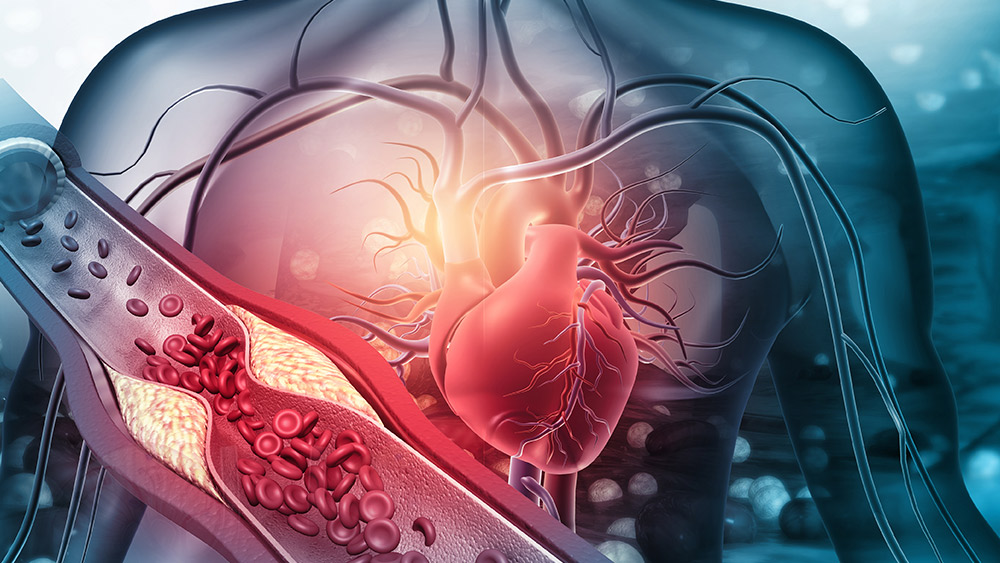
By monitoring and capturing dynamic events, the team can track the progression of atherosclerosis with high physiological relevance and precision that cannot be provided by current preclinical experimental systems. |
Image: Getty Images
Researchers at Texas A&M University are working to design a longer-term organ-on-chip (Vessel-Chip) system, a 3D cell-culture model that mimics living organs' biological activities. This will increase understanding of the progression and signs of atherosclerosis (ACD) — the buildup of fats and/or cholesterol in and on artery walls — and drug-tissue interactions in both astronauts and earthbound humans.
Since age and radiation exposure are powerful risk factors for ACD in both astronauts and earthbound humans, the primary focus of this research is to model these stressors and associated therapeutics by using human-induced pluripotent stem cells (hiPSC) and immune cells.
By monitoring and capturing dynamic events over a period of several months, the team can track the progression of ACD with high physiological relevance and precision that cannot be provided by current preclinical experimental systems.
“For the first time, we will be able to validate our hiPSC-Vessel-Chip for the prolonged modeling of a healthy blood vessel followed by an assessment of cellular and proton radiation stressors and of mRNA therapeutics,” said Dr. Abhishek Jain, associate professor in the Department of Biomedical Engineering. “We want to understand how aging and radiation may result in ACD in both astronauts and earthbound humans.”
The research team includes Dr. John Cooke, Dr. Nhat-Tu Le and Dr. Guangyu Wang at Houston Methodist Research Institute, and Dr. Vladislav Yakovlev, professor of biomedical engineering at Texas A&M.
These Vessel-Chip systems offer a promising in vitro modeling platform where current systems may fall short, including the most relevant hiPSC-derived cells, and the ability to perform long-term investigations all in a clinically relevant hemodynamic microenvironment.
The team plans to achieve these goals in two phases. In the first phase, the researchers will use the existing Vessel-Chip resembling human arterial dimensions and cyclic hemodynamics. They will continuously monitor physiological parameters and sustain the culture of hiPSC-derived endothelial cells (hiPSC-EC) and vascular smooth muscle cells [AD2] (hiPSC-SMC) for a few months.
The plan in the second phase will be to introduce iPSC [AD3] cells from patients with progeria — a genetic disorder causing children to age rapidly — who also exhibit symptoms of ACD. The team will expose the Vessel-Chip to radiation and test novel mRNA therapy in the system for months of observation.
“The organ-on-chip technology of blood vessels has been transformational over the last decade. But in the context of modeling ACD, the significance of our system is the inclusion of hiPSC endothelial cells, smooth muscle cells, immune cells, pulsatile hemodynamics and relevant humoral factors in an anatomical architecture, and tracking performance over a long period,” Jain said. The maximum duration such vessel systems have performed does not exceed two to three weeks, and this system will outperform most others in terms of stability and longevity.
This proposition will produce a platform technology to deploy in future human clinical trials for vascular diseases.
This chip also has broader value because the insights garnered regarding mRNA delivery to the vessel wall will be useful for vascular targeting of other RNA therapeutics going forward, including gene editing.
Another opportunity would be to use hiPSCs from different racial and ethnic patient groups and characterize those differences in response to stressors of vascular health since this disparity in ACD is known.
“An interesting future prospect with this device would be to test how robust the model is when additional subject variables are added to the model, including intraluminal pressure or tobacco condensate from burning tobacco, as these variables are often associated with ACD disease in elderly patients,” Jain said.
This research is jointly funded by NASA, the National Institutes of Health, the Food and Drug Administration and the Biomedical Advanced Research and Development Authority.
Read more about this program on the NASA website.
Since age and radiation exposure are powerful risk factors for ACD in both astronauts and earthbound humans, the primary focus of this research is to model these stressors and associated therapeutics by using human-induced pluripotent stem cells (hiPSC) and immune cells.
By monitoring and capturing dynamic events over a period of several months, the team can track the progression of ACD with high physiological relevance and precision that cannot be provided by current preclinical experimental systems.
“For the first time, we will be able to validate our hiPSC-Vessel-Chip for the prolonged modeling of a healthy blood vessel followed by an assessment of cellular and proton radiation stressors and of mRNA therapeutics,” said Dr. Abhishek Jain, associate professor in the Department of Biomedical Engineering. “We want to understand how aging and radiation may result in ACD in both astronauts and earthbound humans.”
The research team includes Dr. John Cooke, Dr. Nhat-Tu Le and Dr. Guangyu Wang at Houston Methodist Research Institute, and Dr. Vladislav Yakovlev, professor of biomedical engineering at Texas A&M.
These Vessel-Chip systems offer a promising in vitro modeling platform where current systems may fall short, including the most relevant hiPSC-derived cells, and the ability to perform long-term investigations all in a clinically relevant hemodynamic microenvironment.
The team plans to achieve these goals in two phases. In the first phase, the researchers will use the existing Vessel-Chip resembling human arterial dimensions and cyclic hemodynamics. They will continuously monitor physiological parameters and sustain the culture of hiPSC-derived endothelial cells (hiPSC-EC) and vascular smooth muscle cells [AD2] (hiPSC-SMC) for a few months.
The plan in the second phase will be to introduce iPSC [AD3] cells from patients with progeria — a genetic disorder causing children to age rapidly — who also exhibit symptoms of ACD. The team will expose the Vessel-Chip to radiation and test novel mRNA therapy in the system for months of observation.
“The organ-on-chip technology of blood vessels has been transformational over the last decade. But in the context of modeling ACD, the significance of our system is the inclusion of hiPSC endothelial cells, smooth muscle cells, immune cells, pulsatile hemodynamics and relevant humoral factors in an anatomical architecture, and tracking performance over a long period,” Jain said. The maximum duration such vessel systems have performed does not exceed two to three weeks, and this system will outperform most others in terms of stability and longevity.
This proposition will produce a platform technology to deploy in future human clinical trials for vascular diseases.
This chip also has broader value because the insights garnered regarding mRNA delivery to the vessel wall will be useful for vascular targeting of other RNA therapeutics going forward, including gene editing.
Another opportunity would be to use hiPSCs from different racial and ethnic patient groups and characterize those differences in response to stressors of vascular health since this disparity in ACD is known.
“An interesting future prospect with this device would be to test how robust the model is when additional subject variables are added to the model, including intraluminal pressure or tobacco condensate from burning tobacco, as these variables are often associated with ACD disease in elderly patients,” Jain said.
This research is jointly funded by NASA, the National Institutes of Health, the Food and Drug Administration and the Biomedical Advanced Research and Development Authority.
Read more about this program on the NASA website.