T cells are the immune soldiers at the frontlines of the battle with infiltrating pathogens that seek to cause disease. A new study published in Nature Biomedical Engineering describes a novel label-free imaging technique that can differentiate active T cells from those off duty.
The method, developed by scientists at the Morgridge Institute of Research in Wisconsin, could help assess T cell involvement in immunotherapies for cancer treatment or autoimmune diseases.
Dr. Alex Walsh, corresponding author on the paper and assistant professor in the Department of Biomedical Engineering, was an assistant scientist at the Morgridge Institute before joining Texas A&M University.
In a healthy individual, most T cells are in a quiescent state — they’re inactive, but ready and waiting for the signal to join in active combat against an invading virus or bacteria.
“We wanted to test if our imaging technology could tell the difference between the quiescent T cells and activated T cells,” Walsh said.
“T cells have a metabolic switch that regulates their activity,” said Dr. Melissa Skala, principal investigator at the Morgridge Institute and associate professor of biomedical engineering at UW-Madison.
Most methods for characterizing T cells are antibody-based, such as flow cytometry or immunohistochemistry. These require staining with antibodies or contrast agents, a process that is destructive to the cells.
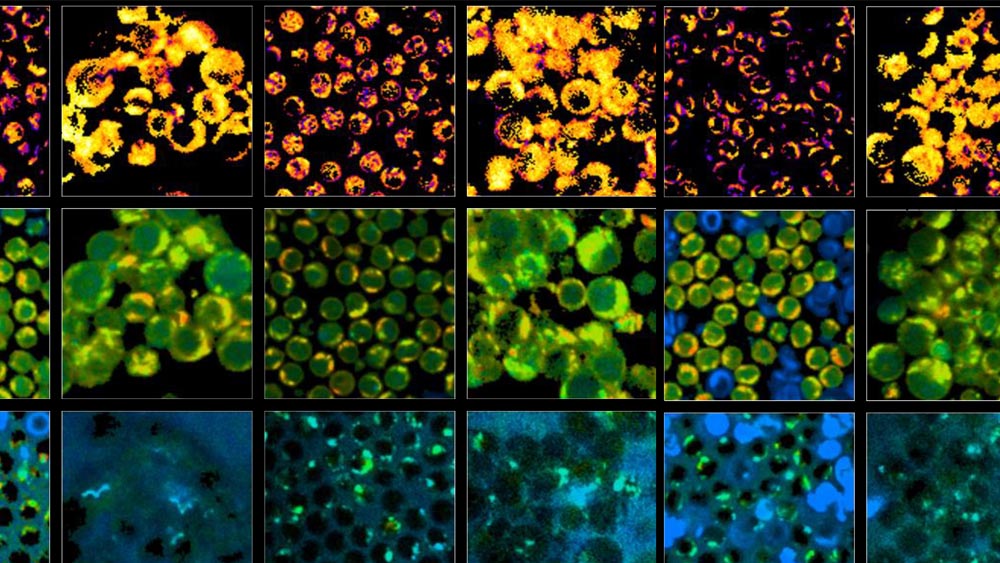
Alternatively, Walsh and Skala’s method detects autofluorescence from molecules within the cell that naturally emit light when imaged by a microscope paired with an infrared laser. This label-free process is non-damaging and doesn’t alter the behavior of the cell. The technique could be adapted to image cells in a plate or dish, tissue samples or even in vivo imaging of a complete organism.
“It's super novel,” Skala said. “Most people aren't using these techniques—you don't see a lot of autofluorescence studies in immunology.”
To validate their approach, the researchers acquired blood samples from healthy donors, isolated the T cells and measured autofluorescence of molecules that are involved in cellular metabolism.
“We kept some of the T cells in their quiescent state, and then we added antibodies to a group to activate them,” Walsh said.
Images of the quiescent cells versus the activated cells revealed differences in metabolic function, most notably through a change in one type of molecule autofluorescence in the activated T cell populations. They also observed that active T cells were slightly larger in size than quiescent cells.
The activation protocol and imaging capabilities will be useful for manufacturing cells used in immunotherapies, said Skala. These re-engineered T cells are often co-cultured with other cells, like cancer cells, to test their reactivity.
The autofluorescent approach provides an attractive way to perform those experiments by imaging the same cells across multiple timepoints in a way that’s non-damaging.
“We showed that you can resolve temporal changes with our imaging technique,” Walsh said. “We were able to see changes in the imaging endpoints within minutes after adding the activating antibodies.”
Walsh adds that it would be difficult to see these dynamic changes using flow cytometry, since the time required for staining and incubation make it difficult to capture multiple timepoints.
While this new technique offers many advantages over traditional methods, there are still limitations. For one, autofluorescence imaging isn’t very sensitive.
“We aren’t relying on really specific labels, we’re relying on the metabolism of the cells,” Skala said. “That’s only going to get you so far in differentiating the cell types.”
Additionally, the technique requires experienced people to perform the microscopic imaging and analyze the data, said Walsh.