Chemical purification and separation are among the most energy intensive processes on Earth. In 2010, chemical purification and separation accounted for around 15 percent of global energy consumption. As many of the separation and purification dependent commodities, such as water, fuel and chemicals, are projected to grow three times by 2050, the same processes will require around 45 – 50 percent of global energy usage. The traditional separation processes are not sustainable and a drastically different process is needed.
Dr. Hae-Kwon Jeong, associate professor in the Artie McFerrin Department of Chemical Engineering at Texas A&M University, has developed a novel method to separate light olefins – such as ethylene and propylene that are used in packaging, plastic processing and textile manufacturing – from paraffins – such as ethane and propane. This is one of the most significant separations in chemical and petrochemical industries, with propylene production amounting to around $90 billion annually worldwide, yet one of the most challenging due to the similarity of their physical and chemical properties. Traditionally, this separation requires the use of massive, energy-intensive distillation columns.
Jeong’s research has focused on the use of membrane-based separations. Essentially, membrane-based separations act much the same as colanders or strainers; a mixture flows through the membrane that allows the smaller molecules through, while excluding the larger molecules. Membrane-based separations are quite common throughout the chemical industry, with the majority of these processes utilizing different polymer membranes. Light olefins and paraffins present a challenge as the size differences between the molecules are around .3 Ångströms (around 0.000000003 centimeters). Polymers are not selective enough to be a useful membrane for this process.
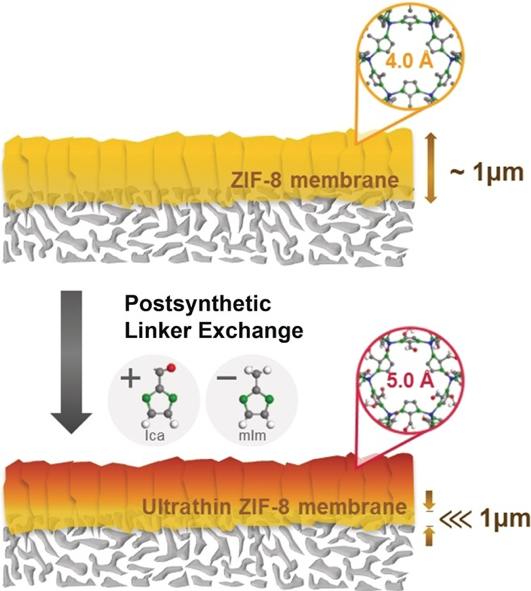
Jeong’s research utilizes metal-organic frameworks (MOFs) as the membrane material, especially Zeolitic-imidazolate frameworks (ZIF), which are composed of metal ions (usually Zn2+ or Co2+) and imidazole-derived organic linkers. Jeong has already shown that ZIF-8 membranes (composed of zinc and 2-methylimidazole) are incredibly effective at separating propylene and propane. However, ZIF-8 membranes are relatively expensive to produce, and a significant improvement to their productivity – essentially the speed with which the molecules are filtered – is required for them have any practical applications. There are two ways increase the productivity of the membrane; reduce the membrane thickness and/or increase the area per volume of the membrane.
Jeong has developed a novel method to drastically reduce the effective size of the ZIF-8 membrane, detailed in a paper published in Angewandte Chemie. Rather than using a membrane composed wholly of ZIF-8, he replaced the top layer of the membrane with ZIF-90. The aperture of ZIF-90 is slightly larger than that of ZIF-8, but they both share the same structure (see image below). Therefore, by adding the top layer of ZIF-90, Jeong was able to reduce the effective thickness of the ZIF-8 membrane, which increased the propylene permeance by about four times with little to no loss in its separation effectiveness. This drastically improves the productivity of ZIF-8 membranes, and opens the door for practical applications.