Plastic is not just pliable and recyclable; research is proving that polymers can be incredibly powerful.

Dr. Jodie Lutkenhaus, associate professor and William and Ruth Neely Faculty Fellow in the Artie McFerrin Department of Chemical Engineering, was recently awarded over $300,000 by the Department of Energy to study electrochemical energy storage in organic radical polymers. In addition to researching energy storage in purely plastic materials, Lutkenhaus creates hybrid polymer-based electrodes that could be used in battery technology.
“For me, plastic power means augmenting conventional batteries with polymers to produce something special you didn’t have before, to create unique properties,” said Lutkenhaus. Mechanical and electrochemical properties within the battery itself are often enhanced by the presence and processing of polymers.
Polymer material selection is closely considered with respect to chemistry and the effects of integration with various forms of battery material. Equally essential to development is the processing that must take place.
“The two materials must come together so they can work synergistically with each other,” said Lutkenhaus. Polymers can be added to energy storage devices to create bendable batteries and structures that endure. Her group's recent publication in Scientific Reports details a highly flexible battery cathode material.
“Our polymer essentially acts like a mechanical glue, so it won’t crack,” said Lutkenhaus. “It will last longer, it’s flexible and it helps the electrochemical reaction take place.”
Lutkenhaus said the expected outcome of the organic radical polymer research is new fundamental knowledge of these “incredibly kinetic polymers” which are currently produced in-house but could be scaled up for industrial purposes.
Inherently safe batteries are the ultimate goal in the years to come.
“We’re also incorporating high-strength nanofibers to make very tough electrodes, ones that might be load-bearing or explosion proof,” said Lutkenhaus.
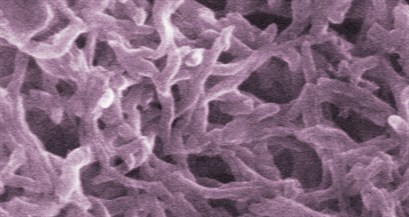
Illustration above: A color-tinted illustration of polyaniline nanofibers