Many people are familiar with the natural compound known as propane, used often as fuel, but less is discussed about propylene (or propene), with which propane is closely tied.
The central focus of the research team led by Dr. Hae-Kwon Jeong, associate professor of chemical engineering, is an optimal isolation of propylene from propane, with the intention to produce only propylene. “There is a huge demand for propylene, but to separate these gas molecules takes a lot of energy,” said Jeong.
Propylene has many commercial purposes, including being a key compound in over two thirds of the world’s plastics. Intentional production of propylene in 2013 accounted for 12 percent of worldwide propylene production, compared to three percent in 2003, according to the IHS Chemical North American Propylene Supply Study. Furthermore, by 2023, the study finds that the amount of intentional global propylene production will grow from 90 million tons to 130 million tons.
In the production process, the gasses are converted into liquid and then distilled, utilizing the slightly different boiling points of propylene and propane. Then, the use of a membrane allows for the smaller propylene to be sieved (restricting the larger propane) in a metal-organic framework (MOF), said Jeong.
“This is the first report where we demonstrate that a very high selectivity and a very high flux of these gas molecules is possible," said Jeong. "This is a simple technique that allows us to prepare high-quality, very-thin sieve MOF membranes in a potentially economic and scalable manner."
Jeong’s method of counter diffusion involves individual applications of metal ions and organic ligands (linkers that bond to the metal) to the support or film, as opposed to using a mixture to coat the film. The high metal ion concentration is inside the film while the ligands remain highly concentrated outside of the film and counter diffuse in different directions.
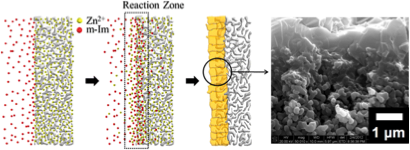
“The objective is to be able to make small, thin films in a rapid manner, but also to be able to make the grain boundary very intimate,” said Jeong.
This technique is not only more efficient, but also more environmentally friendly because less carbon-based fuels are required for distillation. “Up to 80 percent of energy costs go just to separating chemicals, often using conventional distillation,” said Jeong.
Jeong said that if the industry can replace some of the highly intensive energy processes with simple sieving techniques, great progress could be made.
“Opportunities of this research are tremendous. We are getting interest from industry and plan to continue the research,” said Jeong.